Nuclear energy is the energy trapped inside of an atom. Nuclear fission is when an atom's nucleus is split apart releasing an
tremendous amount of energy. The energy released is both light and heat energy.
When a nucleus of an atom fissions it splits into several smaller fragments, usually two different fragments or fission products. The fission products are equal or half the mass of the
original mass of the atom. During the process of fission two or three neutrons are emitted, large quantities of energy is released and radioactive products are formed.
Fission can occur spontaneously or when a nucleus of a heavy atom captures a neutron.
Fissile isotopes are isotopes of an element that can be split through fission. There are only certain isotopes that are fissile (able to fission). An important factor that w
ill determine whether or not isotopes will fission, is the speed of the bombarding neutron. Some isotopes such as
thorium-232
requires a very fast moving neutron for fission, where as uranium-235 needs a slow moving neutron to fission. If a neutron is too fast it will pass right through the atom which will not affect the isotope at all.

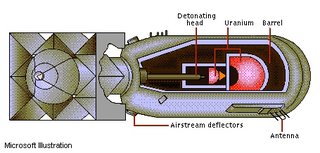
 5. When a neutron strikes uranium-235 it is first absorbed making uranium-236, and then it becomes unstable causing the atom to fission. The fissioning of uranium-236 can create many new products such as: 235U + 1 neutron ---> 2 neutrons + 92Kr+ 142Ba +Energy 235U + 1 neutron ---> 2 neutrons + 92Sr + 142Xe +Energy In both of these reactions the neutron splits the atom and when the atom splits another neutron is released. This is how a chain reaction may occur, because if there was more uranium-235 present those two neutrons could cause two more atoms to split. Each of those two atoms will release an additional neutron, resulting in a total of four neutrons. These four neutrons would strike more uranium-235 atoms releasing even more neutrons which causes a chain reaction to continue until all uranium-235 is gone.
5. When a neutron strikes uranium-235 it is first absorbed making uranium-236, and then it becomes unstable causing the atom to fission. The fissioning of uranium-236 can create many new products such as: 235U + 1 neutron ---> 2 neutrons + 92Kr+ 142Ba +Energy 235U + 1 neutron ---> 2 neutrons + 92Sr + 142Xe +Energy In both of these reactions the neutron splits the atom and when the atom splits another neutron is released. This is how a chain reaction may occur, because if there was more uranium-235 present those two neutrons could cause two more atoms to split. Each of those two atoms will release an additional neutron, resulting in a total of four neutrons. These four neutrons would strike more uranium-235 atoms releasing even more neutrons which causes a chain reaction to continue until all uranium-235 is gone. 

 Otto Hahn and Fritz
Otto Hahn and Fritz 